Specific heat is a numeric value which refers to the amount of heat required to raise a set quantity of a substance by a fixed temperature. For example, pure water has a specific heat of 1. This means one calorie will raise the temperature of one gram of water by one degree C. Or, in U.S. units, one BTU will raise the temperature of one pound of water by one degree F.
Note that regardless of the system of units used, the numeric value of a substance's specific heat is the same. And all other substances are referenced to the specific heat of water which, as already stated, is 1. For example, oil has a typical, specific heat of 0.51. This means it takes only 51% of the heat to raise the temperature of oil as it does an equal quantity of water.
Based on this property alone, water appears to be a superior hydraulic fluid to oil. Because it takes twice is much heat, to get the same quantity of water to the same temperature. However, specific heat cuts both ways. Meaning, once water does get to a certain temperature, twice as much heat must be removed to drop the same quantity by the same temperature, when compared with oil.
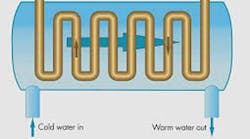
The difference in specific heat between water and oil is leveraged in oil-to-water heat exchangers. Because one pound of water only has to increase in temperature by one degree F to cool two pounds of oil by one degree F. But regardless of the type of heat exchanger employed, not keeping the hydraulic oil cool can be one of the costliest maintenance mistakes you can make. And to discover six other costly mistakes you want to be sure to avoid with your hydraulic equipment, get "Six Costly Mistakes Most Hydraulics Users Make... And How You Can Avoid Them!" available for FREE download here.
Sponsored Recommendations
May 15, 2024
March 13, 2024
March 13, 2024
March 13, 2024