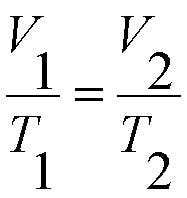What’s the Difference Between Pascal’s, Boyle’s, and Charles’ Laws?
Boyle’s, Charles’, and Pascal’s Laws describe the basic behavior of fluids with respect to volume, pressure, and temperature. Here’s a quick look at all three.
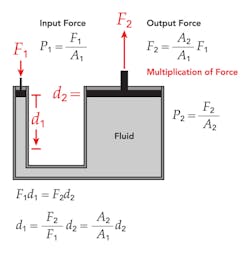
Pascal’s Law was established around 1648 by Frenchman Blaise Pascal. It states that pressure applied anywhere in a confined incompressible fluid is transmitted equally in all directions throughout that fluid.
Pascal's principle applies to a confined incompressible fluid. One typical application can be found in most automotive repair shops that have a lift. Basically, air from a compressor is applied to the top of the oil in a sealed container. The oil then applies pressure to a sleeve/piston that lifts the car. This is the same principle used in hydraulic jacks. However, the smaller hydraulic cylinder must travel much farther than the larger lift cylinder. This lets the jack lift a heavy load with a small force, as in an auto hydraulic lift—but of course there can be no multiplication of work, so in an ideal case with no friction loss.
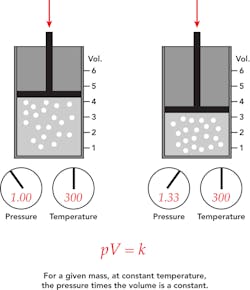
Boyle’s Law, a basic law in chemistry, describes the behavior of a gas held at constant temperature. The law, discovered by Robert A. Boyle in 1662, states that at a fixed temperature, the volume of gas is inversely proportional to the pressure exerted by the gas. In other words, when a gas is pumped into an enclosed space, it will shrink to fit into that space, but the pressure that gas puts on the container will increase.
Perhaps a more straightforward explanation is to say Boyle’s law is the relationship between pressure and volume. Mathematically, Boyle’s law can be written as pV=k, where p is the pressure of the gas, V is the volume of the gas, and k is a constant.
An example of Boyle’s law in action can be seen in a balloon. Air is blown into the balloon; the pressure of that air pushes on the rubber, making the balloon expand. If one end of the balloon is squeezed, making the volume smaller, the pressure inside increases, making the un-squeezed part of the balloon expand. There is a limit to how much the air/gas can be compressed, however, because eventually the pressure becomes so great it breaks the balloon.
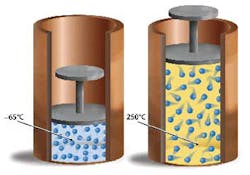
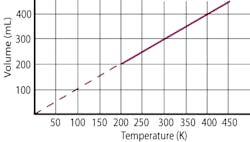
Charles’ Law was first formulated by Joseph Louis Gay-Lussac in 1802, but he credited it to unpublished work by Jacques Charles. It states that if pressure remains constant, the volume of a mass of gas is proportional to its absolute temperature. The absolute temperature is always 273 Kelvin more than the centigrade temperature.
A useful expression of this physics law is:
Where V1 is the volume of a mass of gas at temperature T1 (in kelvins) and V2 is its volume after the temperature has changed to T2 (in kelvins).
Another illustration that is helpful for understanding Charles’ Law is when you have the same amount of molecules in two separate volumes, yet they displace different volumes because they are at two different temperatures.
The graph below plots the formula and shows either the volume or temperature as they change.
If you have any questions regarding the behavior of gases and fluids, please feel free to contact Clippard’s Technical Support team online or by calling (877) 245-6247.